Inside This Issue: HIV diagnosis rates and social vulnerability, new resources on HIV among Black Americans, gender-affirming care and treatment, updated HIV treatment guidelines, PrEP research plan, nPEP toolkit, and more.
Black Americans and HIV
CDC Study Finds Higher HIV Diagnosis Rates Among Black Americans in Communities with High Social Vulnerability
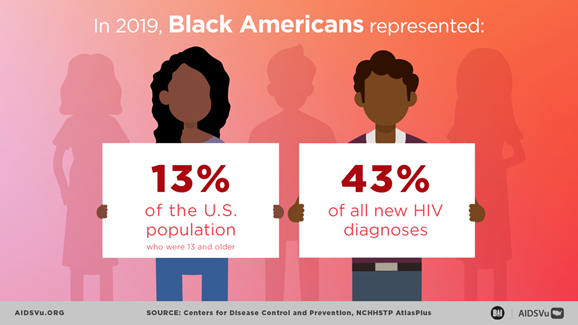
During 2019, Black Americans accounted for more than 40% of all new HIV diagnoses in the United States. The annual diagnosis rate among Black persons was about eight times higher than that in White persons and ten times higher than in persons of Asian descent. To better understand the factors that might contribute to this “profound disparity in HIV diagnoses,” CDC researchers used National HIV Surveillance System (NHSS) and Social Vulnerability Index (SVI) data to examine the association between diagnosed HIV infections and social vulnerability among Black adults age 18 years or older. “Social vulnerability” refers to the potential negative health effects on communities caused by external stresses. CDC’s SVI ranks U.S. Census tracks on 15 social factors, including poverty, lack of vehicle access, and crowded housing, and groups them into four related themes: socioeconomic status; household composition and disability; minority status and language; and housing type and transportation.
The CDC researchers found that the HIV diagnosis rate of Black adults living in communities in the highest quartile of SVI was 1.5 times higher than among Black adults in communities in the lowest quartile of SVI. “Because of a history of racial discrimination and residential segregation, some Black persons in the United States reside in communities with the highest social vulnerability, and this finding is associated with experiencing increased risk for HIV infection,” the researchers note. “The development and prioritization of interventions that address social determinants of health (i.e., the conditions in which persons are born, grow, live, work, and age), are critical to address the higher risk for HIV infection among Black adults living in communities with high levels of social vulnerability. Such interventions might help prevent HIV transmission and reduce disparities among Black adults.”
AIDSVu Publishes Blog Items and Infographics Focusing on HIV Among Black Americans
Shortly before National Black HIV/AIDS Awareness Day (NBHAAD) on February 7, AIDSVu published three blog items highlighting the impacts of HIV on Black Americans. Dr. Rueben Warren, director of the National Center for Bioethics in Research and Health Care, writes on the bioethics of HIV disparities in the Black community, as well as the impact of the history of racial disparities in health care on COVID-19 vaccine hesitancy. In another blog item, Reginald Smith, director of the Reginald & Dionne Smith Foundation, describes his experience as a Black heterosexual man living with HIV and the efforts of his foundation to promote total wellness for families affected by HIV, hepatitis, and substance use disorders.
The third recent AIDSVu blog item marking NHBAAD includes a series of updated infographics focusing on HIV testing, diagnoses, trends, regional and age distribution of new diagnoses, and viral suppression among Black Americans:
- Relatively High Prevalence of HIV Among Black Americans
- New HIV Diagnoses Among Black Americans in the South
- Proportion of New Diagnoses Among Black Americans During 2020
- Disproportional Impact of HIV on Young Black Men
- Recent Trends in HIV Diagnoses Among Black Americans
- Viral Suppression Rates Among Black Americans Compared to Persons of Other Races/Ethnicities
- Variation in HIV Testing Rates by Race/Ethnicity
For additional information on this topic, you may visit NEAETC’s Online HIV Resource Library page focusing on Blacks/African Americans and HIV.
%201.jpg)
Transgender Persons and HIV
HRSA Promotes Access to Gender-Affirming Care and Treatment in RWHAP
In a recent letter, the Health Resources and Services Administration (HRSA) HIV/AIDS Bureau (HAB) encouraged Ryan White HIV/AIDS Program (RWHAP) service providers “to harness and mobilize the existing RWHAP infrastructure and services to support gender-affirming services within allowable RWHAP parameters.” The letter notes that RWHAP funds may be used to support gender-affirming care across various RWHAP core medical and support service categories. This includes access to gender-affirming hormone therapy, as well as purchasing and maintaining private health insurance, Medicaid, and Medicare coverage, which can support a broad range of health needs for transgender people with HIV.
“In addition, RWHAP recipients and subrecipients may provide behavioral and mental health services to clients experiencing gender dysphoria and social and emotional stress related to transgender discrimination, stigma, and rejection. RWHAP funds may be used to provide housing, case management, and substance use disorder treatment services, which are fundamental in reducing health disparities and improving HIV-related outcomes among transgender people. RWHAP AIDS Education and Training Centers provide training and education to clinicians and healthcare staff on cultural humility, cultural sensitivity, and inclusive care for diverse populations. HRSA HAB funds activities that support patient-centered, trauma-informed, and inclusive environments of care for RWHAP recipients through training, technical assistance, and other initiatives to reduce barriers to antiretroviral therapy adherence and maximize the likelihood of achieving viral suppression.”
For additional information on this topic, you may visit NEAETC’s Online HIV Resource Library page focusing on Transgender People and HIV.
%202%20-%20small.jpeg)
HIV Treatment
HHS Updates HIV Treatment Guidelines for Adults and Adolescents
In late January, an expert panel updated the U.S. Department of Health and Human Services (HHS) Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. Highlights are summarized below:
Acute and Recent HIV Infection: In the previous version of the guidelines, the expert panel suggested that an HIV viral load under 10,000 copies/milliliter (mL) in a person suspected of having acute HIV infection might be a false-positive test result. However, the panel has changed its view, taking into consideration that current HIV RNA tests have greater sensitivity and specificity than in the past. The panel now notes that a person exposed to HIV or with symptoms compatible with acute infection may indeed have acute infection even if they have low HIV RNA levels (under 3,000 copies/mL) in the setting of a negative or indeterminate HIV antibody test result. The panel also noted, however, that in rare cases, an HIV RNA level below 3,000 copies/mL may represent a false-positive quantitative test result. In such cases, the panel recommends repeat testing to confirm the diagnosis. In addition, the revised treatment guidelines provide updated information about the diagnosis of acute HIV in persons receiving pre-exposure prophylaxis and subsequent initiation of antiretroviral therapy (ART).
Discontinuation or Interruption of ART: This section of the guidelines has been updated to include discussions about discontinuing or interrupting long-acting antiretroviral drugs, including ibalizumab and the intramuscular formulations of cabotegravir and rilpivirine. It also includes guidance on steps to take before and during ART interruption for people with HIV who participate in clinical trials that involve analytical treatment interruptions.
%20small.jpeg)
Pre- and Post-Exposure Prophylaxis
USPSTF Publishes Research Plan for HIV PrEP
The U.S. Preventive Services Task Force (USPSTF) has finalized its research plan on pre-exposure prophylaxis (PrEP) for HIV prevention. The plan identifies five main research questions concerning:
- the benefits of PrEP for HIV prevention and quality of life, including possible differences in benefits among various population groups, as well as for different dosing strategies and regimens;
- the relative efficacy of newer PrEP regimens, compared to the original PrEP regimen tenofovir disoproxil fumarate-emtricitabine (TDF-FTC);
- the accuracy of provider or patient risk assessment tools in identifying PrEP candidates;
- the potential harms of PrEP compared to placebo or no PrEP when used for HIV prevention; and
- the potential harms of newer PrEP regimens compared to TDF-FTC.
The research plan also discusses the analytical framework that the USPSTF will use, the contextual questions that will be considered, its research approach, and the additions and changes made to its draft PrEP research plan in response to public comments.
For additional information on this topic, you may visit NEAETC’s Online HIV Resource Library page focusing on PrEP.
New nPEP Toolkit Available from AETC NCRC
The AIDS Education & Training Center (AETC) National Coordinating Resource Center (NCRC) recently posted a new Non-Occupational Post-Exposure Prophylaxis (nPEP) Toolkit to its website. The toolkit has four components:
nPEP Quick Guide for Providers – This summarizes assessment, treatment, and follow-up recommendations for people with known or potential exposures to HIV and other infections.
nPEP Myths and Facts Flyer – This dispels six common myths about nPEP, including when to start nPEP, who can prescribe it, and potential side effects, among others.
Prescribing nPEP: Key Concepts – This poster provides essential information about early initiation of nPEP, HIV testing before starting nPEP, starting treatment, emphasizing adherence, possible side effects, and scheduling follow-up appointments for counseling and monitoring.
Medication Assistance Program Quick Guide – This web page has contacts for public, non-profit, and manufacturer-operated programs to help patients pay for medications, including those used for PEP.
For additional information on this topic, you may visit NEAETC’s Online HIV Resource Library page focusing on PEP.
Other Educational Resources
Updated Fact Sheets from Clinicalinfo.hiv.gov
HHS has recently updated five main resource pages on its clinicalinfo.hiv.gov English-language and Spanish-language web pages. For your convenience, we have provided links to these resource pages, together with a list of fact sheets that may be accessed there.
HIV and Opportunistic Infections, Coinfections, and Conditions – This topic area includes six fact sheets on HIV comorbidities, including what opportunistic infections are, hepatitis B, tuberculosis, sexually transmitted diseases, heart disease, and kidney disease.
Living with HIV – This includes four fact sheets on finding treatment services, mental health, nutrition and food safety, and substance use.
HIV and Special Populations – This includes three fact sheets focusing on children and adolescents, women, and gay and bisexual men.
HIV Treatment – This includes nine fact sheets on HIV treatment basics, steps after testing positive for HIV, when to start HIV medicines, choosing an HIV regimen, HIV drug resistance, treatment adherence, following an HIV treatment regimen, drug interactions, and immunizations.
Side Effects of HIV Medicines – This includes seven fact sheets about side effects, diabetes, hepatotoxicity, high cholesterol, lactic acidosis, osteoporosis, and rash.
Summary of ONAP Listening Session on HIV Criminalization
HIV.gov recently published a summary of a January 25 virtual listening session on HIV criminalization laws convened by the White House’s Office of National AIDS Policy (ONAP). Participants in the meeting included legal, academic, and public health experts, as well as persons with HIV. Community members suggested ways to eliminate or reform outdated HIV criminalization laws, and recommended actions that various stakeholders could take now to help reduce or halt prosecutions under existing HIV criminalization laws in 35 states. “The discussion was rich and surfaced a variety of issues, concerns, and potential opportunities for further exploration,” noted Harold Phillips, senior HIV advisor and chief operating officer for ONAP’s Ending the HIV Epidemic initiative. “This was an extremely valuable meeting that will inform the way ONAP approaches these issues moving forward.”
COVID-19 Vaccination
Moderna’s COVID-19 Vaccine Becomes the Second to Gain FDA Approval
On January 31, the U.S. Food and Drug Administration (FDA) approved Moderna’s COVID-19 vaccine for the prevention of COVID-19 in persons 18 years or older. The vaccine, which will be marketed under the name Spikevax, is the second to receive FDA approval. The agency initially granted emergency use authorization (EUA) for the Moderna vaccine on December 18, 2020. Spikevax has the same formulation as the EUA Moderna COVID-19 Vaccine and is administered as a primary series of two doses, one month apart.
The Moderna COVID-19 Vaccine remains available under its EUA as a two-dose primary series for persons aged 18 years or older, as a third primary series dose for persons 18 years or older who are immunocompromised, and as a single booster dose for persons 18 years or older at least five months after they complete a primary series of the vaccine. The vaccine is also authorized for use as a “mix and match” single booster dose for persons 18 years or older who have completed primary vaccination with a different COVID-19 vaccine.
“The FDA’s approval of Spikevax is a significant step in the fight against the COVID-19 pandemic” noted Dr. Janet Woodcock, acting FDA commissioner. “While hundreds of millions of doses of Moderna COVID-19 Vaccine have been administered to individuals under EUA, we understand that for some individuals, FDA approval of this vaccine may instill additional confidence in making the decision to get vaccinated.”
Other COVID-19 News
Recent Data Summaries and Research Reports
CDC’s COVID Data Tracker Weekly Review highlights key data from its COVID Data Tracker, narrative interpretations of the data, and visualizations from the week. Themes of recent Weekly Review issues include early detection (including wastewater monitoring) and “staying up to date” (receiving all initial recommended doses of vaccines plus boosters).
The Morbidity and Mortality Weekly Report (MMWR) is also providing continuing coverage of COVID-19-related research. CDC is archiving its MMWR reports on a page devoted to studies about COVID-19. For your convenience, we have compiled links to recent COVID-19 reports below, organized by subtopic. These reports were published in MMWR unless otherwise noted.
Vaccine Approvals and Recommendations
- Coronavirus (COVID-19) Update: FDA Takes Key Action by Approving Second COVID-19 Vaccine (FDA)
- Use of the Janssen (Johnson & Johnson) COVID-19 Vaccine: Updated Interim Recommendations from the Advisory Committee on Immunization Practices – United States, December 2021
HIV and COVID-19
- Notes from the Field: COVID-19 Vaccination Among Persons Living with Diagnosed HIV Infection – New York, October 2021
- Notes from the Field: HIV Outbreak During the COVID-19 Pandemic Among Persons Who Inject Drugs – Kanawha County, West Virginia, 2019-2021
For additional information on this topic, you may visit NEAETC’s Online HIV Resource Library page focusing on COVID-19 and HIV.
Omicron Variant
- Trends in Disease Severity and Health Care Utilization During the Early Omicron Variant Period Compared with Previous SARS-CoV-2 High Transmission Periods – United States, December 2020-January 2022
- Notes from the Field: Early Evidence of the SARS-CoV-2 B.1.1.529 (Omicron) Variant in Community Wastewater – United States, November-December 2021
Vaccination Coverage, Efficacy, and Health Outcomes
- COVID-19 Vaccination Coverage and Vaccine Confidence by Sexual Orientation and Gender Identity – United States, August 29-October 30, 2021
- SARS-CoV-2 Infection and Hospitalization Among Adults Aged ≥18 Years, by Vaccination Status, Before and During SARS-CoV-2 B.1.1.529 (Omicron) Variant Predominance – Los Angeles County, California, November 7, 2021-January 8, 2022
- Effectiveness of a Third Dose of Pfizer-BioNTech and Moderna Vaccines in Preventing COVID-19 Hospitalization Among Immunocompetent and Immunocompromised Adults – United States, August-December 2021
- COVID-19 Cases and Hospitalizations by COVID-19 Vaccination Status and Previous COVID-19 Diagnosis – California and New York, May-November 2021
- Effectiveness of a Third Dose of mRNA Vaccines Against COVID-19-Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Adults During Periods of Delta and Omicron Variant Predominance – VISION Network, 10 States, August 2021-January 2022
- COVID-19 Incidence and Death Rates Among Unvaccinated and Fully Vaccinated Adults with and Without Booster Doses During Periods of Delta and Omicron Variant Emergence – 25 U.S. Jurisdictions, April 4-December 25, 2021




%20small.jpg)
.jpeg)