Inside This Issue: News on COVID-19 vaccine effectiveness and vaccination of young children; HIV prevention and treatment in U.S. health centers; HRSA funding awards to health centers; the global HIV epidemic, educational resources, and other COVID-19 news.

COVID-19 Vaccination
CDC: Even After Rise of Delta Variant, Vaccines Remained Highly Effective in Preventing Hospitalization and Death
Even after Delta became the predominant COVID-19 variant in the U.S., vaccination continued to provide substantial protection against COVID-19 infection, serious illness requiring hospitalization, and death, according to a recent report published in the Morbidity and Mortality Weekly Report. To determine what effect the rise of the Delta variant had on vaccine effectiveness, researchers from CDC and state and local agencies analyzed data on the incidence of COVID-19 cases, hospitalizations, and deaths among vaccinated and unvaccinated persons during the period from April 4-July 17, 2021. The researchers also compared data for two sub-periods: April 4-June 19, when the prevalence of the Delta variant was under 50%; and June 20-July 17, when the prevalence of the Delta variant was 50% or more.
The data analysis showed that, even after the Delta variant became most common, COVID-19 vaccination reduced the risk greater than 10-fold for both hospitalization and death, and reduced the risk of infection about 5-fold. However, the incidence rate ratio (IRR) for new COVID-19 cases decreased substantially – an indication that COVID-19 vaccines were less effective in preventing SARS-CoV-2 infection. The effectiveness of vaccines in preventing new SARS-CoV-2 infection decreased from about 91% in the April 4-June 19 period before the Delta variant predominated, to 78% during the June 20-July 17 period after.
“The framework used in this analysis allows for comparisons of observed IRRs and percentages of vaccinated cases, hospitalizations, and deaths to expected values,” the researchers concluded. “The data might be helpful in communicating the real-time impact of vaccines (e.g., persons not fully vaccinated having >10 times higher COVID-19 mortality risk) and guiding prevention strategies, such as vaccination and nonpharmacologic interventions.”
FDA Outlines Approach for COVID-19 Vaccination of Young Children
“As schools around the country are re-opening for in-person learning and families are returning to their busy school year schedules, we know many parents are anxious about the pandemic and protecting their children,” begins a recent statement from the U.S. Food and Drug Administration (FDA) outlining the agency’s approach for evaluating and authorizing the use of COVID-19 vaccines in children under 12 years old. The statement – attributed to Acting FDA Commissioner Dr. Janet Woodcock, and Dr. Peter Marks, director of the FDA’s Center for Biologics Research and Evaluation – addresses common questions and concerns about COVID-19 Vaccination in young children.
“It’s important that the public recognize that, because young children are still growing and developing, it’s critical that thorough and robust clinical trials of adequate size are completed to evaluate the safety and the immune response to a COVID-19 vaccine in this population,” Drs. Woodcock and Marks note. “Children are not small adults – and issues that may be addressed in pediatric vaccine trials can include whether there is a need for different doses or different strength formulations of vaccines already used for adults.”
The FDA statement outlines the following steps the agency is taking to ensure the safety and efficacy of COVID-19 vaccines in children:
- Vaccine manufacturers must complete ongoing vaccine trials in pediatric patients, including a two-month follow-up period to allow for proper safety monitoring.
- The manufacturers must then complete a data analysis of their vaccines’ safety and efficacy in children. FDA will work with manufacturers to ensure that their data analysis is robust and meets regulatory standards. Then manufacturers may request an emergency use authorization (EUA) or approval of a biologics license application (BLA) for use of the vaccine in children.
- After requests from manufacturers are received, FDA must thoroughly review the data to evaluate each vaccine’s benefits and risks in children before making its decision about granting an EUA or BLA approval.
“Just like every vaccine decision we’ve made during this pandemic, our evaluation of data on the use of COVID-19 vaccines in children will not cut any corners,” Drs. Woodcock and Marks note. “Conducting clinical trials to determine an appropriate vaccine dose in children requires additional work over that done in the adult studies, including ensuring that the vaccine dosage and formulation strength used is the appropriate one from the perspective of safety and generating an immune response.”
U.S. HIV Epidemic
HIV Prevention and Treatment in Health Centers During 2020
Last month, the Health Resources and Services Administration (HRSA) Bureau of Primary Health Care (BPHC) released calendar year 2020 data for its Health Center Program. BPHC Associate Administrator James Macrae summarized HIV-specific highlights for health centers in a recent blog item on the HIV.gov site. Overall, health centers conducted almost 2.5 million HIV tests; delivered primary care services to nearly 190,000 patients with HIV; provided pre-exposure prophylaxis (PrEP) services to 389,000 patients; and helped ensure that 81% of newly diagnosed HIV-positive persons between the ages of 15 and 65 years old were linked to care within 30 days of diagnosis.
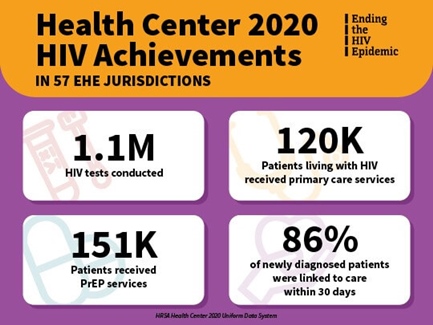
Macrae also noted that the 195 health centers that received FY2020 Primary Care HIV Prevention (PCHP) awards contributed substantially to health centers’ overall achievements in providing HIV-related services nationwide. Specifically, these PCHP-funded health centers:
- conducted nearly 1.1 million HIV tests (44% of overall HIV tests);
- delivered primary care services to more than 120,000 patients living with HIV (63% of total patients);
- provided PrEP services to over 151,000 patients (40% of the total patients receiving PrEP-related services); and
- ensured that 86% of newly diagnosed HIV-positive persons were promptly linked to care (outperforming the overall health center rate by 5 percentage points).
HIV testing and primary care visits decreased 11% and 3%, respectively, during 2020 – likely the result of COVID-19-related travel restrictions and clinic closures, which resulted in fewer in-person visits overall for health centers. Macrae noted that, “PCHP-funded health centers have shown resilience and dedication in serving our nation’s most vulnerable and at-risk populations, and we are extremely proud of their efforts in the Ending the HIV Epidemic initiative.”

Funding Awards
HRSA Awards Health Centers Nearly $50 Million in Support of EHE Initiative
On September 16, HRSA awarded more than $48 million to 271 HRSA-supported health centers across 26 states, Puerto Rico, and the District of Columbia to expand HIV prevention and treatment, including PrEP-related services, outreach, and care coordination. This investment is part of the Ending the HIV Epidemic (EHE) initiative, which has the goal of reducing the number of new U.S. HIV infections 90% by 2030.
This funding will expand HIV prevention services that reduce the risk of HIV transmission in counties, territories, and states prioritized due to their high burden of HIV. This year’s HRSA Health Center Program EHE awards include funding to additional health centers in EHE high-priority jurisdictions. “HHS-supported community health centers are often a key point of entry to HIV prevention and treatment services, especially for underserved populations,” noted Xavier Becerra, Secretary of the U.S. Department of Health and Human Services. “Today’s awards will ensure equitable access to services free from stigma and discrimination, while advancing the Biden-Harris Administration’s efforts to end the HIV/AIDS epidemic by 2025.”
Global HIV Epidemic
UNAIDS: Global HIV Treatment Has Saved Millions of Lives, But Falls Short of 2020 Targets
The United Nation’s 90-90-90 targets for HIV, adopted in 2016, called for 90% of all people with HIV to be tested and diagnosed; 90% of persons diagnosed to start HIV treatment, and 90% of those on treatment to achieve HIV viral suppression by 2020. Achieving these targets would require that at least 73% of people with HIV have suppressed viral loads. This is equivalent to about 27.5 million of the estimated 37.7 million people with HIV worldwide in 2020.
Although many nations have made substantial progress toward ending HIV/AIDS, the 2020 global targets have not yet been met, according to a recent update from UNAIDS. The agency estimates that, at the end of 2020, 84% of people with HIV (about 31.7 million) knew their HIV status, 73% (about 27.5 million) were accessing antiretroviral therapy, and 66% (about 24.9 million) were virally suppressed.
Nevertheless, the global roll-out of HIV treatment has averted an estimated 16.5 million AIDS-related deaths since 2001, according to UNAIDS. In 2020, there were approximately 680,000 deaths from AIDS-related causes, a decline of 58% from 2001 to 2020. At least 40 countries are on track to achieve a 90% reduction in AIDS-related mortality by 2030, including nine countries in eastern and southern Africa.
Global Fund Report Details “Devastating Impact” of COVID-19 on HIV, TB, and Malaria Programs
The COVID-19 pandemic had a devastating impact on the fight against HIV, tuberculosis (TB), and malaria in 2020, according to a new report released by the Global Fund. “The Global Fund partnership continues to save lives,” noted Peter Sands, Fund executive director. “But the 2020 numbers . . . confirm what we feared might happen when COVID-19 struck.”
The report indicates that, while some progress was made, several key programmatic results declined for the first time in the 20-year history of the Fund. The COVID-19 pandemic led to significant declines in HIV testing and prevention services for key and vulnerable populations who were already disproportionately affected. Compared with 2019, the number of people reached with HIV prevention programs and services declined by 11% overall, including a 12% decrease among young people. The number of women receiving medicine to prevent mother-to-child HIV transmission fell by 4.5%. HIV testing dropped by 22%, which limited HIV antiretroviral treatment (ART) initiation in most countries that receive support from the Fund. Medical male circumcision for HIV prevention also declined by 27%.
In addition, during 2020, the number of people treated for drug-resistant TB in the countries where the Global Fund invests dropped 19%. The decline was nearly twice as large – 37% – among persons receiving treatment for extensively drug-resistant TB. The number of HIV-positive TB patients receiving both antiretroviral treatment and TB treatment also decreased 16%.
Despite the challenges of the COVID-19 pandemic, countries where the Fund invests achieved the following results in 2020:
- 9 million people received ART, an 8.8% increase compared to 2019;
- 7 million people were reached with HIV prevention services;
- 7 million people were treated for TB;
- 194,000 children in contact with patients exposed to TB received preventive therapy; and
- 188 million mosquito nets were distributed to protect families from malaria, a 17% increase compared to 2019.
Educational Resources
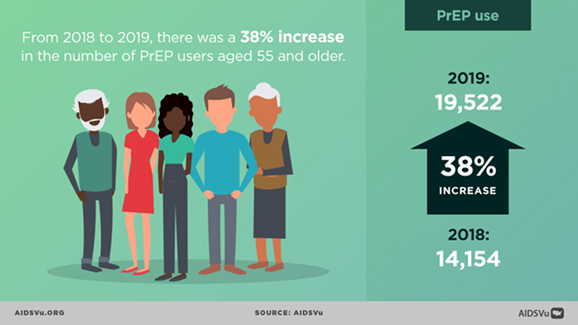
New AIDSVu Infographics Focus on HIV Among Older Persons
In the run-up to National HIV/AIDS and Aging Awareness Day last week, AIDSVu published a blog item summarizing recent statistics on HIV among people aged 55 years or older in the U.S., as well as the following infographics, updated with 2019 data, focusing on this age group:
- The HIV Care Continuum Among People Aged 55 and Older
- U.S. Map Showing the Number of Older Persons with HIV by County
- New HIV Diagnoses and Deaths Among Older Persons by Race/Ethnicity
- Women Account for 30% of New HIV Diagnoses Among Older Persons
- Recent Increase in HIV PrEP Use Among Older Persons
- The PrEP-to-Need Ratio Among Older Persons
.jpeg)
Other COVID-19 News
CDC’s Updated COVID Recommendations for Healthcare Personnel Include Specific Guidance for Dental and Dialysis Facilities and EMS
Earlier this month, CDC published the latest update to its Interim Infection Prevention and Control Recommendations for Healthcare Personnel During the Coronavirus Disease 2019 (COVID-19) Pandemic. The updated document includes guidance on the following:
- infection prevention and control practices for fully vaccinated HCP, patients, and visitors;
- testing for SARS-CoV-2 infection;
- use of Duration of Transmission-Based Precautions for patients with SARS-CoV-2 infection; and
- considerations for specialized healthcare settings, such as dental and dialysis facilities and Emergency Medical Services (EMS). To find the specific recommendations for these settings, you can visit the recommendations page and search for “dental,” “dialysis,” or “Emergency Medical Services.”
Mark Schweizer, DDS, also summarized COVID-19 recent dental news, recommendations, and resources in a post on the AETC National Coordinating Resource Center’s ShareSpot blog.
For an annotated list and links to additional COVID-19 guidelines, recommendations, and other materials, please check out NEAETC’s COVID-19 Resources Page.
Recent Data Summaries and Research Reports
CDC’s COVID Data Tracker Weekly Review highlights key data from CDC’s COVID Data Tracker, narrative interpretations of the data, and visualizations from the week. This online newsletter also summarizes important trends in the pandemic and brings together CDC data and reporting in a centralized location. The themes for the three most recent issues were: Vaccinations and Variants; An Ounce of Prevention; and Testing, Testing: School Edition.
CDC’s Morbidity and Mortality Weekly Report (MMWR) is also providing continuing coverage of COVID-19-related research. CDC is archiving the MMWR reports on a page devoted to studies about COVID-19. For your convenience, we have compiled links to recent MMWR papers below:
Long-Term Symptoms Among Adults Tested for SARS-CoV-2 – United States, January 2020-April 2021
Using Wastewater Surveillance Data to Support the COVID-19 Response – United States, 2020-2021




%20small.jpg)